Biography
Research during my PhD and post-doctorate period
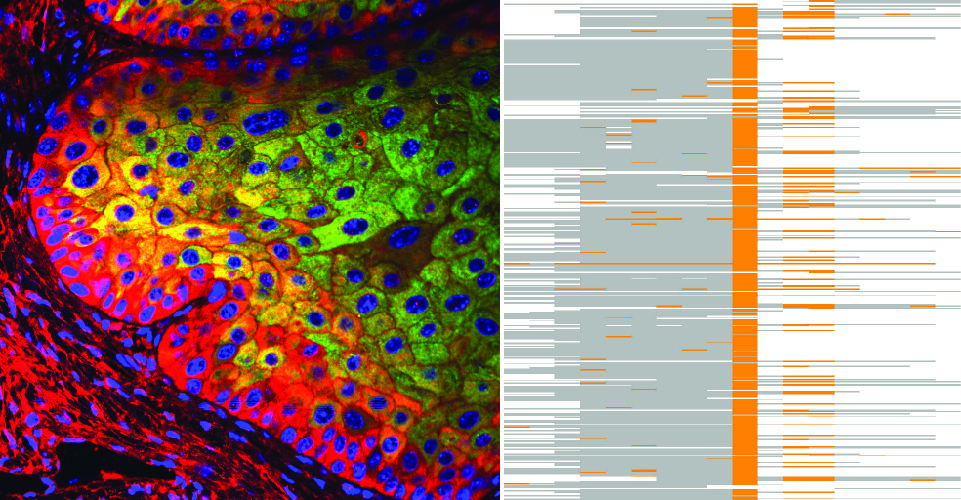
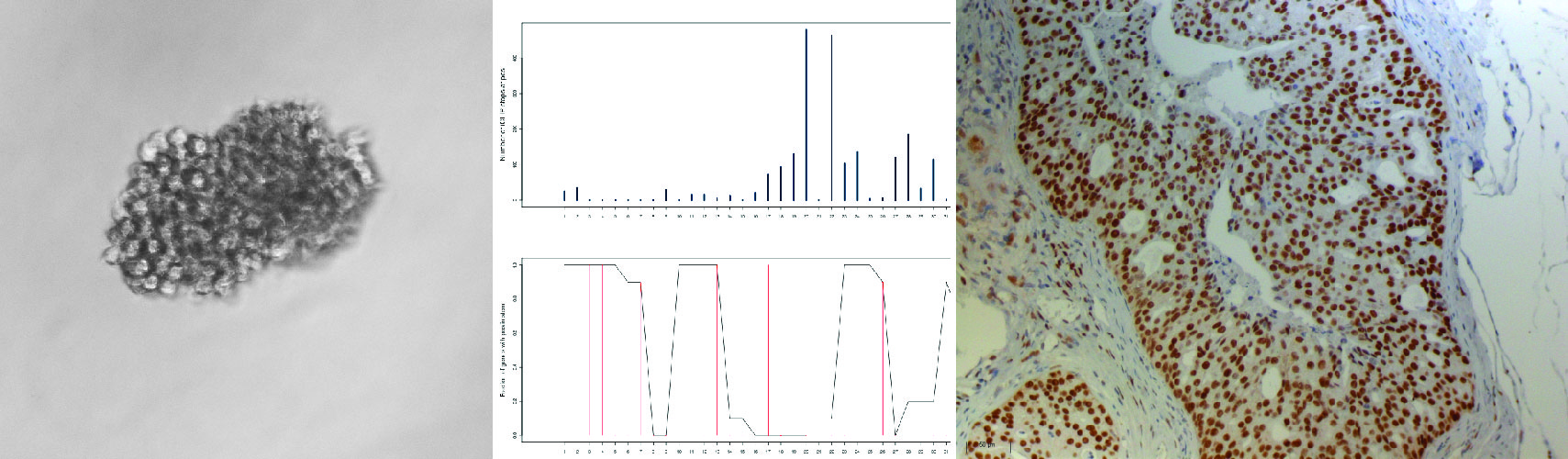
During my PhD and my post-doctorate, I have been engaged in finding the molecular mechanisms that lead to cancer. Initially during my PhD, at the Institute of Molecular and Cellular Biology of Cancer (Salamanca, Spain), I studied post-translation modifications (phosphorylation), and particularly dysfunctional kinases as possible targets for cancer therapy under the supervision of Prof. Pedro A. Lazo. During my post-doctorate, in the group of Dr. Michaela Frye at the Wellcome Trust – MRC Cambridge Stem Cell Institute – University of Cambridge, I studied the functional role of post-transcriptional RNA modifications in tissue homeostasis and the impact of their dysregulation in diseases such as cancer. My work largely contributed to the knowledge we have gained to date on the functional role of post-transcriptional modifications and pathological outcomes of their dysregulation, in particular 5-methylcytosine deposition in RNA. There are around 100 known covalent RNA modifications, yet our knowledge about their occurrence and physiological function in mammals is still very limited. My studies have demonstrated that cytosine-5 RNA methylation has an essential role in cellular processes including self-renewal and stress responses in tissue and cancer stem cells.
By combining stem cell biology and mouse genetics, I found that cytosine-5 RNA methylation (m5C) is a novel mechanism by which stem and progenitor cells (both in tissue and in cancer) balance self-renewal and differentiation/proliferation properties 1-5. By using novel transcriptome‐wide sequencing approaches together with mouse genetics, I determined that cytosine-5 RNA methylation is a widespread modification in coding, non-coding RNAs and mainly transfer RNAs (tRNAs) 1,6-10 which regulated self-renewal in tissue stem cells but also sensitivity to stress in tumour initiating cells 4,5,9. Thus, my work in the laboratory of M. Frye was innovative and shed light on novel dysfunctional and targetable molecular pathways in cancer. Our findings were pioneers in exploring yet unknown mechanisms in stem cell and cancer biology and established the emergence of a novel research field coined as the Epitranscriptome. Moreover, our studies were the first to mechanistically link altered RNA methylation and cancer, and set novel road maps to chart the discovery of novel therapeutic strategies to treat cancer 5,11,12.
After my postdoctoral position, I was awarded a Ramón y Cajal fellowship (Sept’2016) that enabled my establishment as a Junior PI at CIC bioGUNE (Spain). Since then my research has focused on epitranscriptomics in cancer, in particular defining the molecular traits that the fluctuating epitranscriptome may confer to cancer cells and cancer initiating cells.
References
1 Blanco, S, Kurowski, A, Nichols, J, Watt, FM, Benitah, SA & Frye, M. The RNA-methyltransferase Misu (NSun2) poises epidermal stem cells to differentiate. PLoS genetics 7, e1002403, doi:10.1371/journal.pgen.1002403 (2011).
2 Hussain, S, Tuorto, F, Menon, S, Blanco, S, Cox, C, Flores, JV, Watt, S, Kudo, NR, Lyko, F & Frye, M. The mouse cytosine-5 RNA methyltransferase NSun2 is a component of the chromatoid body and required for testis differentiation. Molecular and cellular biology 33, 1561-1570, doi:10.1128/MCB.01523-12 (2013).
3 Blanco, S & Frye, M. Role of RNA methyltransferases in tissue renewal and pathology. Current opinion in cell biology 31C, 1-7, doi:10.1016/j.ceb.2014.06.006 (2014).
4 Flores, JV, Cordero-Espinoza, L, Oeztuerk-Winder, F, Andersson-Rolf, A, Selmi, T, Blanco, S, Tailor, J, Dietmann, S & Frye, M. Cytosine-5 RNA Methylation Regulates Neural Stem Cell Differentiation and Motility. Stem Cell Reports, doi:10.1016/j.stemcr.2016.11.014 (2016).
5 Blanco, S, Bandiera, R, Popis, M, Hussain, S, Lombard, P, Aleksic, J, Sajini, A, Tanna, H, Cortes-Garrido, R, Gkatza, N, Dietmann, S & Frye, M. Stem cell function and stress response are controlled by protein synthesis. Nature 534, 335-340, doi:10.1038/nature18282 (2016).
6 Martinez, FJ, Lee, JH, Lee, JE, Blanco, S, Nickerson, E, Gabriel, S, Frye, M, Al-Gazali, L & Gleeson, JG. Whole exome sequencing identifies a splicing mutation in NSUN2 as a cause of a Dubowitz-like syndrome. Journal of medical genetics 49, 380-385, doi:10.1136/jmedgenet-2011-100686 (2012).
7 Hussain, S, Aleksic, J, Blanco, S, Dietmann, S & Frye, M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome biology 14, 215, doi:10.1186/gb4143 (2013).
8 Hussain, S, Sajini, AA, Blanco, S, Dietmann, S, Lombard, P, Sugimoto, Y, Paramor, M, Gleeson, JG, Odom, DT, Ule, J & Frye, M. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell reports 4, 255-261, doi:10.1016/j.celrep.2013.06.029 (2013).
9 Blanco, S, Dietmann, S, Flores, JV, Hussain, S, Kutter, C, Humphreys, P, Lukk, M, Lombard, P, Treps, L, Popis, M, Kellner, S, Holter, SM, Garrett, L, Wurst, W, Becker, L, Klopstock, T, Fuchs, H, Gailus-Durner, V, Hrabe de Angelis, M, Karadottir, RT, Helm, M, Ule, J, Gleeson, JG, Odom, DT & Frye, M. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. The EMBO journal 33, 2020-2039, doi:10.15252/embj.201489282 (2014).
10 Van Haute, L, Dietmann, S, Kremer, L, Hussain, S, Pearce, SF, Powell, CA, Rorbach, J, Lantaff, R, Blanco, S, Sauer, S, Kotzaeridou, U, Hoffmann, GF, Memari, Y, Kolb-Kokocinski, A, Durbin, R, Mayr, JA, Frye, M, Prokisch, H & Minczuk, M. Deficient methylation and formylation of mt-tRNA(Met) wobble cytosine in a patient carrying mutations in NSUN3. Nature communications 7, 12039, doi:10.1038/ncomms12039 (2016).
11 Frye, M & Blanco, S. Post-transcriptional modifications in development and stem cells. Development 143, 3871-3881, doi:10.1242/dev.136556 (2016).
12 Popis, MC, Blanco, S & Frye, M. Posttranscriptional methylation of transfer and ribosomal RNA in stress response pathways, cell differentiation, and cancer. Current opinion in oncology 28, 65-71, doi:10.1097/CCO.0000000000000252 (2016).


 Ana Macrina Añazco Guenkova
Ana Macrina Añazco Guenkova Aurora Campos Díaz
Aurora Campos Díaz Raquel García Vilchez
Raquel García Vilchez Judith López Luís
Judith López Luís Domenico Rosace
Domenico Rosace Víctor de Frutos Herrero
Víctor de Frutos Herrero